Background
Rechargeable batteries are driving the global energy transition to renewable energy, and it is foreseeable that battery production will increase significantly in the coming years. However, conventional Li-ion batteries rely on transition metal oxide materials such as cobalt and nickel oxides as cathodes because of their high energy density and long cycle life. As demand for lithium-ion batteries is expected to surge, there could be significant consumption of these transition metals, creating potential supply problems. The availability of transition metal resources is limited, resulting in high production costs, and their large-scale use is neither sustainable nor environmentally sound. In addition, transition metal materials currently account for nearly 30-50% of the global warming impact in battery manufacturing. As a result, various alternative approaches have been proposed that allow us to move away from traditional transition metal-based active materials and, in turn, achieve sustainable battery chemistry.
Organic rechargeable batteries have emerged as a promising option for sustainable energy storage because they utilize transition metal-free active materials, i.e. redox-active organic materials, mostly composed of earth-rich carbon, oxygen, hydrogen, and nitrogen. composition. Fundamental research on redox activity in organic compounds has led to the discovery of several important groups of materials with electrochemical performance comparable to traditional transition metal-based materials. Given these potential advantages, tremendous efforts have been made in the past decades to incorporate these redox-active organic materials as key electrode components. Recently, many studies have applied these redox organic electrodes to practical battery systems and achieved performance levels comparable to those of commercial transition-metal-containing batteries. Furthermore, some general disadvantages of organic materials, such as their low electronic conductivity and high solubility, can be effectively mitigated by advanced electrode designs, or translated into technical advantages by modifying the structure of the battery.
This review provides an overview of recent advances in organic rechargeable battery technology, focusing on practical aspects. In addition to providing a comparative evaluation of individual redox-active materials, a series of more pragmatic evaluation dimensions are presented. Then, alternative platforms for redox-active materials in post-Li-ion battery systems are discussed, including the inclusion of earth-abundant metal ions and the development of water-based organic batteries and organic redox flow batteries. This review aims to provide a comprehensive and practical assessment of current challenges and solutions to help further develop sustainable and affordable organic rechargeable batteries.
Assessing redox-active organics
Although the use of transition metal-free active materials is attractive from a sustainability perspective, the precise evaluation of redox-active organic materials is challenging compared to commercial mass-produced transition metal-based electrode materials performance, especially in terms of the actual cost of each performance and environmental benefit. These problems stem from a lack of mass production and the associated economies of scale. Nonetheless, using some relevant indices, such as the current market price and global warming potential (GWP) of typical active materials, a rough estimate can be made. GWP is an index that refers to the amount of carbon dioxide (CO2) or gas equivalent to CO2 produced per kilogram of a particular product produced over a certain period of time. Figure 1a compares the GWP100 values (GWP of 100) of representative cathode materials, LiCoO2 (LCO) and LiNi0.6Co0.2Mn0.2O2 (NCM622), with several large-scale organic compounds, methanol (CH3OH). Olefins (ethylene (C2H4), propylene (C3H6)) and common aromatics (benzene, toluene and mixed xylenes). Commercial transition metal-containing cathode materials clearly have a large impact on greenhouse gas emissions, producing nearly 20 kilograms of carbon dioxide for every kilogram of cathode produced. Although the GWP100 value of future redox-active organic materials is not known, it can be estimated from the GWP100 value of common precursors and the corresponding increase in GWP100 for each additional process. Even though complex industrial organic chemicals typically require two to four steps of additional chemistry starting from precursors, current large-scale production of simple organic compounds yields less than one-fifth the GWP100 of transition metal-containing cathode materials. This value indicates that the estimated GWP of most redox-active organic materials is still significantly lower than that of transition metal materials, as depicted in Fig. 1a. Furthermore, this prediction is conservative, which means that some redox-active organic materials that require simpler synthesis conditions will have much lower GWP100 values.
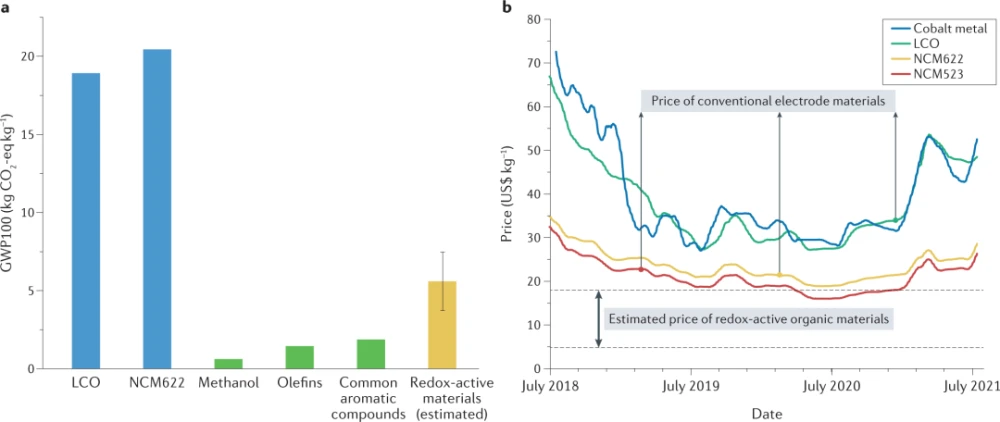
Figure 1: Comparative analysis of the environmental and economic advantages of redox-active organic materials.
Figure 1b presents price changes for representative commercial cathode materials over the past 3 years. Costs fluctuate significantly even over a short period of time, with prices changing more than 2-fold during this period, which is closely related to the cost of primary metals such as cobalt (blue line in Figure 1b). Transition metal-based cathode materials typically account for 40% of the total battery cost, so their price volatility poses an unpredictable risk factor for the mass production of lithium-ion batteries. What’s more, the long-term supply of key transition metals remains fragile and may not be able to cope with the rapid growth of the market. According to recent reports, the lithium-ion battery market is expected to reach a capacity of 3TWh by 2030, roughly 20 times the current capacity (approximately 140GWh in 2020). Based on current cathode chemistry, a 3TWh lithium-ion battery capacity would require about 2.2 million tons of transition metals (cobalt or nickel). Considering that the current annual output of cobalt is 140,000 tons and the annual output of nickel is 2.5 million tons, the demand for transition metals to achieve this 3TWh capacity is not easy to meet. According to the United States Geological Survey (USGS), this demand will become a serious problem in the near future, which will create competition with other industries. The main demand for nickel from the competing stainless steel manufacturing industry can significantly raise the price of nickel during a growing battery market. Contrary to these predictions of transition metal supply and associated costs (Fig. 1b), previous studies have clearly shown that redox-active organic materials will cost less than $15 per kilogram if produced on a large scale via traditional organic synthesis routes. We note that this price was estimated in 2017. This analysis implies that redox-active organic materials are more economical than cobalt-based (LME, 2021) and other transition metal-based materials (Shanghai Metal Market, 2021) for large-scale production of TWh-class batteries potential.
Redox Active Organic Materials
Redox-active organic materials are generally classified as n-type, p-type, or bipolar based on their ability to release electrons (oxidation) or accept electrons (reduction) in the neutral state during electrochemical reactions. N-type redox-active organic materials typically undergo reduction from their neutral state to form a negatively charged molecular state that can be reversed during oxidation. In contrast, electrochemical reactions of p-type organic materials involve oxidation from their neutral state, forming a reversible positively charged molecular state. Some organic compounds are known to undergo bipolar reactions, utilizing redox groups capable of both n-type and p-type behavior. The fundamental redox properties of redox-active organic materials depend on the redox group, a functional group in the molecule that confers redox activity. In the following, the main redox groups constituting representative redox-active organic materials are briefly introduced, as shown in Fig. 2.
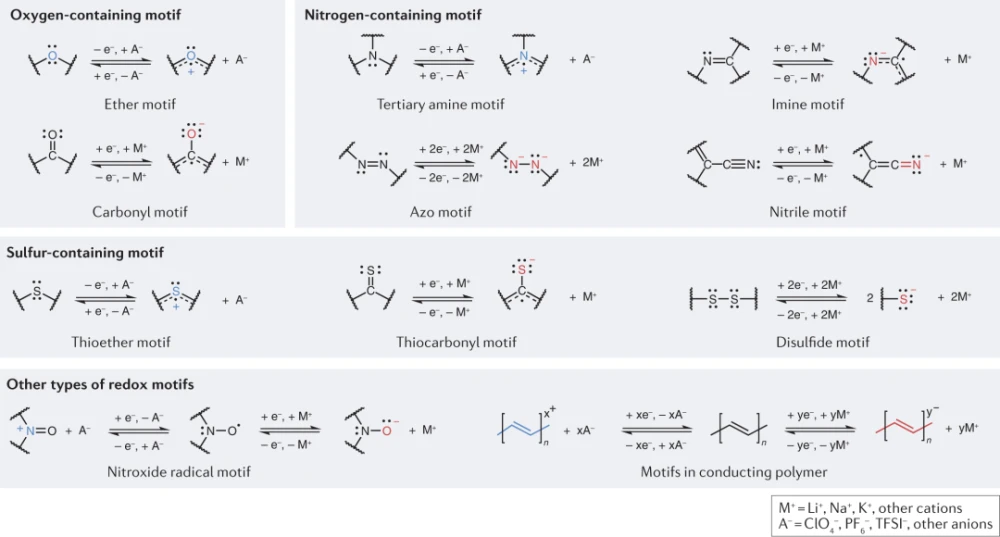
Figure 2: Redox diagrams in representative redox-active organic electrodes.
oxygen-containing group
The most common redox groups in redox-active organic materials are oxygen-containing units, which include two main functional groups: carbonyl and ether. Since the first report of carbonyl organic materials as active materials in 1969, various carbonyl redox-active organic materials have been investigated, including quinone derivatives, carboxylates, imides and acid anhydrides. The carbonyl pattern (C=O) in these materials is capable of undergoing n-type redox reactions while reversibly forming a radical monoanion (C-O−) via enolation. Quinone derivatives, such as vitamin K, ubiquinone, and pratoquinone, are the most widely studied carbonyl compounds because of their abundance in nature and their critical role in biochemical electron transport. Organic materials with carbonyl groups typically provide a discharge potential (vs. Li+/Li) of around 2.5 V, which is relatively low for cathodes and too high for anode applications. However, carboxylic acid compounds, with similar C=O bonds in their molecular structure, can provide low potentials (vs. Li+/Li ~1V), comparable to commercial anode materials such as Li4Ti5O12.
The ether group (C-O-C) promotes a high-potential p-type redox reaction, upon which radical cations are reversibly formed. Organic electrodes with ether-based patterns usually offer the highest redox potentials (vs. Li+/Li, over 4 V) because of their strong electronegativity. However, the utilization of ether-based materials, such as benzoxazine derivatives, as high-potential electrodes is often hindered by the limited electrochemical window of conventional electrolytes and the complexity of anion binding in p-type redox reactions.
Nitrogen containing group
Nitrogenous redox themes include tertiary amine, azo, imine, and nitrile redox centers (Figure 2). The ability of nitrogen atoms to form three covalent bonds makes their redox groups more diverse than their oxygen-containing counterparts. Among them, tertiary amine groups allow similar p-type reactions with ether groups, thus allowing high redox potentials in excess of 3 V in electrode materials based on dihydrophenolic, triphenylamine, phenothiazine and phenoxazine derivatives ( vs. Li+/Li). In particular, dihydrophenolazine-based electrodes are capable of redox reactions of two electrons per molecule, providing high specific capacity, thereby delivering specific energy comparable to commercial lithium iron phosphate (LFP) cathodes.
Azo compounds (N=N), imines (C=N). Nitriles (C≡N) and sulfonamides (C-N(SO2)-) are based on double or triple bonds between nitrogen and carbon, and usually exhibit n-type activity through changes in bond order during redox reactions. Given their relatively simple structure and ability to react reversibly with two alkali ions, the family of azo compounds has a high capacity.
Amines frequently appear in biological charge transfer systems, such as pteridine and pyridine derivatives. They generally consist of heteroaromatic rings with nitrogen atoms in the conjugated structure, and can effectively simplify or modify the molecular structure in various ways, including cutting out the conjugated molecule and leaving only the nitrogen-containing conjugated ring.
For nitrile redox motives, 7,7,8,8-tetracyanodimethane and its derivatives are the most studied because they possess the highest redox potentials among n-type redox active organic materials (2.4–3.2 V vs. Li+/Li). The high redox potential of nitrile compounds can be attributed to the strong electron-accepting properties originating from the four electron-withdrawing nitrile groups. Sulfonamide groups—the latest discovery in this class of compounds—show higher redox potentials than nitrile groups because of electron delocalization on the sulfonyl group attached next to the nitrogen center. Redox-active organic materials with sulfonamide groups are usually synthesized in the original negatively charged reduced form (i.e., combined with cations, such as Li+ or Na+); thus, the initial electrochemical process involves extraction of cations to achieve n-type reactions .
Sulfur group
Sulfur-containing redox groups have similar redox properties to oxygen-containing redox units, probably because sulfur and oxygen are in the same group in the periodic table. A representative thioether group (C-S-C) consists of two single bonds between a sulfur atom and a carbon atom, which usually initiate p-type reactions with a redox mechanism similar to that of ether groups. Thiocarbonyl (C=S) is similar to carbonyl. However, since sulfur is less electronegative than oxygen, it induces an increase in redox potential and enhanced electron conductivity. Disulfides (C-S-S-C) contain a single bond between two sulfur atoms with a unique n-type redox mechanism; the S-S bond is broken after accepting two electrons, and each R-S− anion reacts at a relatively low redox The potential (~2.2V vs. Li+/Li) is stabilized by cations. These compounds can exhibit high specific capacities due to the multi-electron redox per motive, and thus have been intensively studied. However, intrinsically sluggish kinetics originating from S–S bond cleavage or formation during reduction and oxidation lead to high overpotentials. The solubility of small molecules resulting from bond cleavage reactions also leads to premature capacity fading caused by the loss of active species.
Other types of redox groups
Other types of redox groups occur as free radicals or in conducting polymers. One of the most representative examples of this class is the compound based on 2,2,6,6-tetramethyl-1-piperidinyl (TEMPO), which contains a nitroxide radical (N-O-). They are ambipolar in nature, but are more often used as cathode materials, selectively exploiting p-type reactions to take advantage of the associated high redox potential (approximately 3.6 V vs. Li+/Li). TEMPO-based compounds are often reported in the form of polymers because of their high solubility and can exhibit high rate capability due to their self-exchange reactions, i.e., fast intermolecular electron transfer processes. However, large redox-active moieties in polymers generally yield low specific capacities. Likewise, conducting polymers based on polyacetylene, polythiophene, or polyaniline have also been investigated as redox-active materials. Incorporation of counter ions into these polymer electrodes should enable redox reactions to theoretically yield high capacities. However, to achieve these predicted capacities, high doping levels are required, which limits cycling stability due to side reactions of the dopants.
Applications in rechargeable batteries
So far, 56 redox-active organic materials have been reported to possess the aforementioned redox groups (Fig. 3). When incorporated into organic electrodes, these materials offer the most pronounced electrochemical performance in redox-active organics. Regarding the applicability of these candidate materials for practical electrodes, which include not only active materials but also several other components, it should be noted that their performance is mainly evaluated in the literature in traditional transition metal-based battery evaluation frameworks . Furthermore, given that each reported material employs different electrode fabrication procedures, comparative assessments are not straightforward. These issues imply that the intrinsic capabilities of redox-active organic materials may not be properly assessed. The unique properties of organic materials (organics 1 to 45 in Figure 3) in terms of key battery performance metrics—specific energy, specific power, and cycling stability—are discussed below in full consideration of their practical applicability. Methods to exploit the unique properties of organic electrodes to help them surpass the electrochemical performance of conventional lithium-ion batteries are also described.
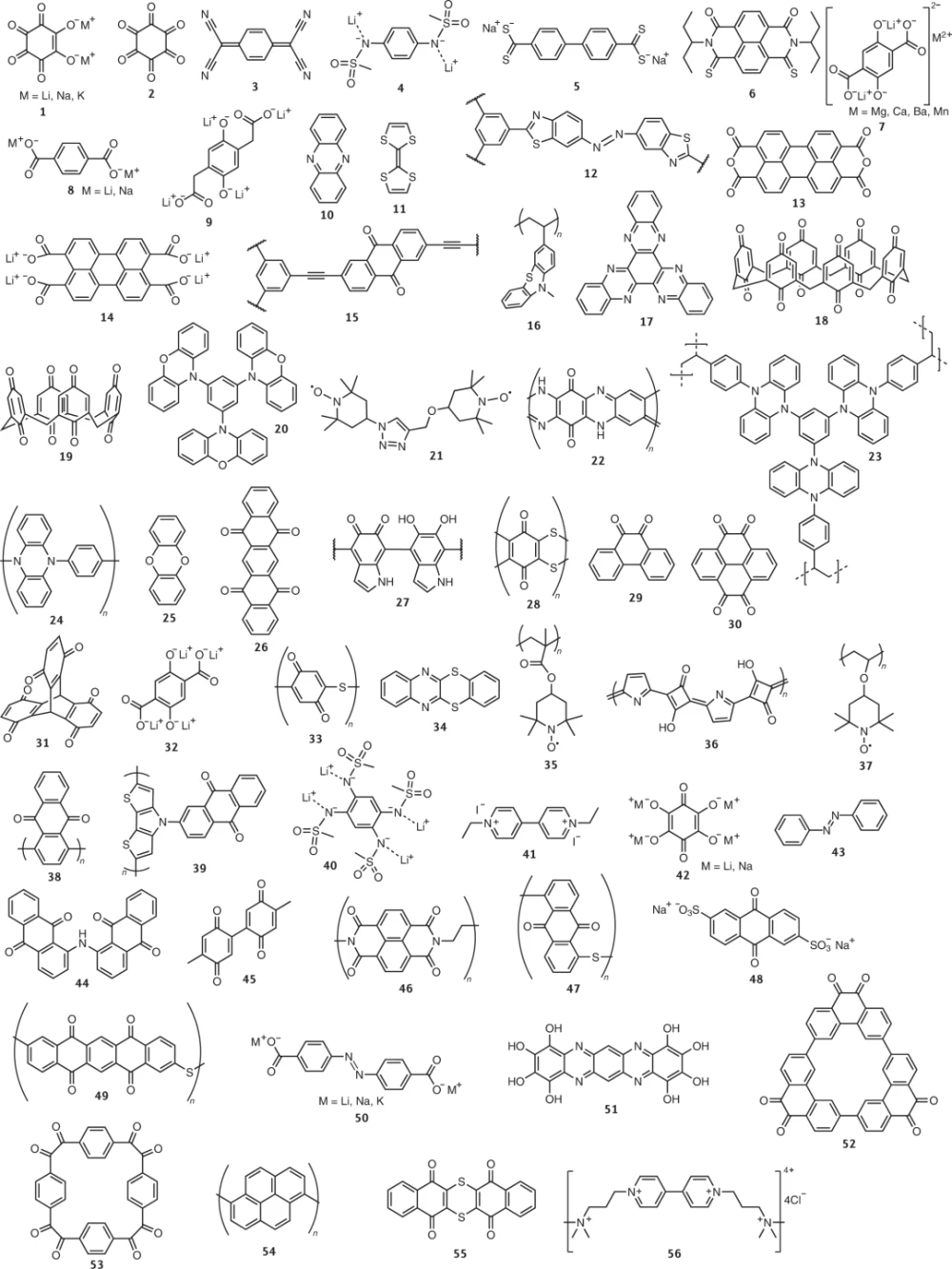
Figure 3: Molecular structures of representative redox-active organic materials. Representative redox-active organic materials exhibiting high battery performance. Their redox properties are generally determined by their redox mechanisms.
Comparative analysis of specific energy
The specific energy is determined by the numerical product of the specific capacity of the active material and the redox potential. The specific capacities and voltages (vs. Li+/Li) of state-of-the-art organic electrode materials as well as conventional transition metal electrode materials are compared in Fig. 4a. The specific capacity and voltage values provided are actual values obtained from discharge curves in the literature. We note that the specific capacities in Fig. 4 and Fig. 5 are estimated from the weight of each material in the ready-to-charge state (i.e., the discharged state) to allow a fair comparison with the specific capacities of transition metal-based inorganic materials. Since the specific capacities of the introduced p-type materials do not take into account the accompanying anions, in some cases the available capacities may vary depending on the electrolyte system, such as in dual-ion systems. Some organic electrode materials, such as LCO, NCM, and LFP, are able to provide two to three times larger capacity than transition metal-based electrode materials due to their lightweight constituent elements and multi-electron transfer capability. Electrodes based on rhodium (organic 1 or cyclohexanone (organic 2) derivatives allow four or six electron transfers per molecule, respectively, resulting in a capacity of approximately 503 mAhg−1 or 723 mAhg−1 and an energy density of 1117 Whkg−1 or 1229 Whkg−1. This is a clear benefit of organic electrode materials over conventional layered transition metal oxide cathodes, which are rapidly reaching their theoretical limit of 270 mAhg−1 due to their high content of transition metal elements.
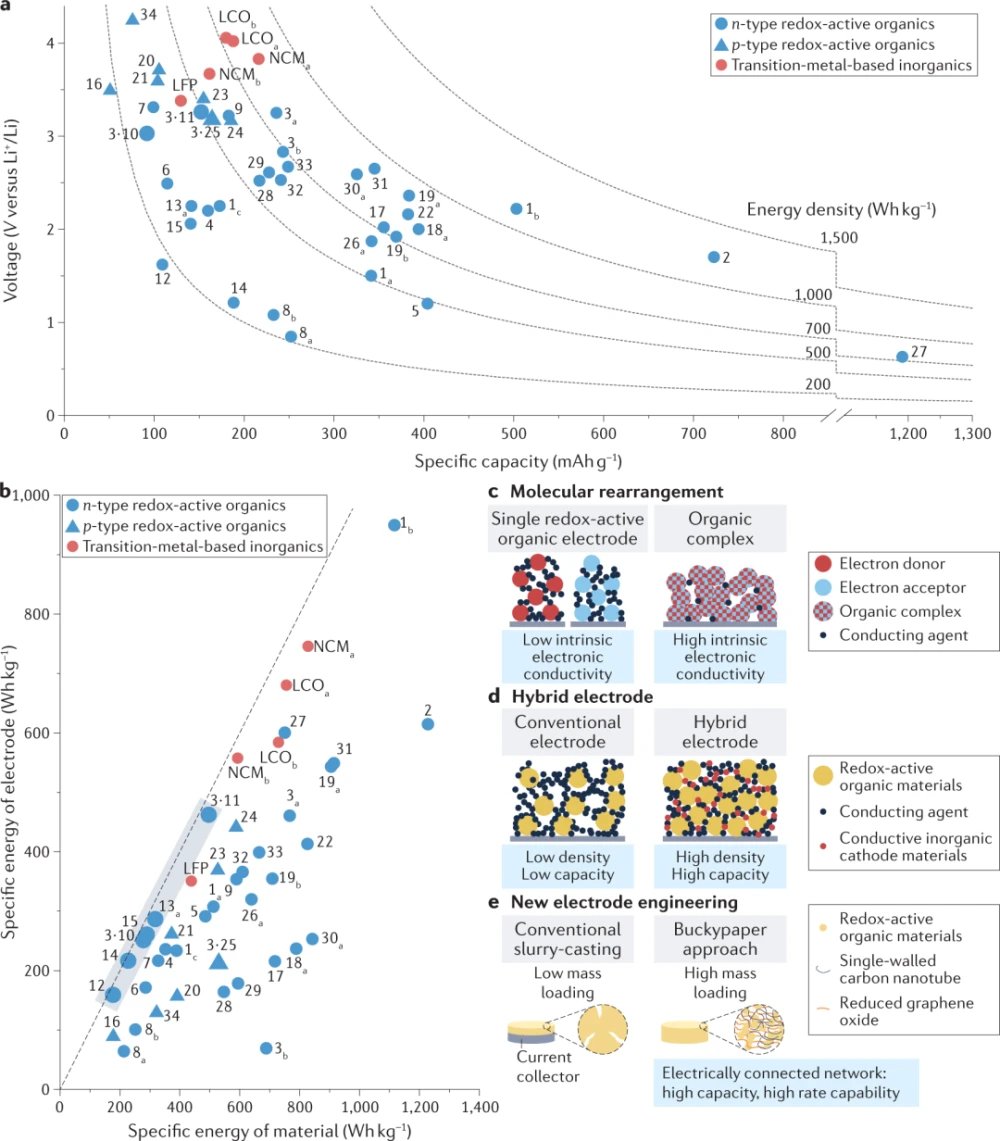
Figure 4: Comparative analysis of specific energies of representative redox-active organic materials.
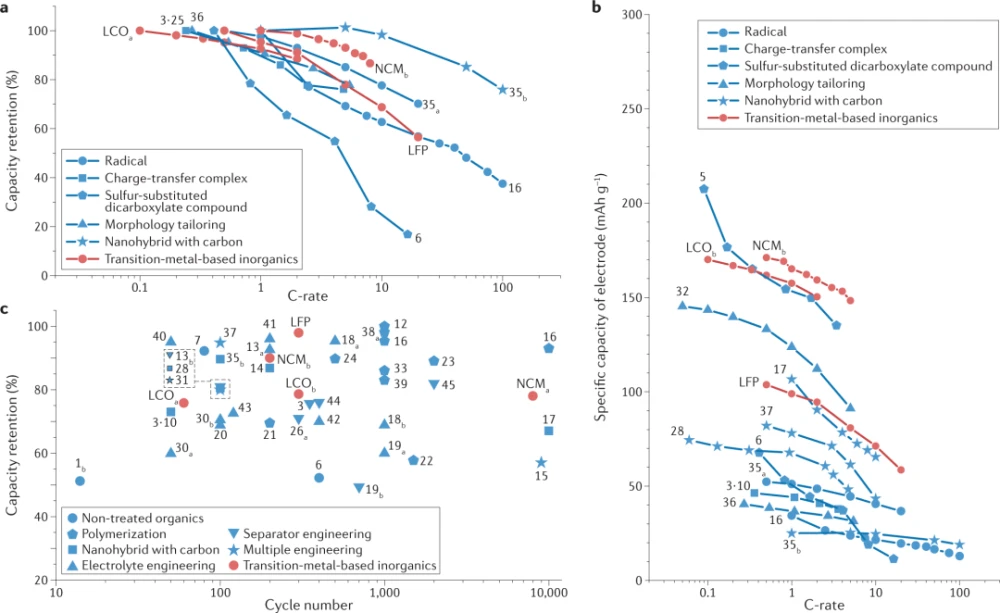
Figure 5: Comparative analysis of rate capability and cycling performance.
Strategies to improve the specific energy of redox-active organic materials
As shown in Figure 4, the high specific capacity of organic electrodes usually comes at the expense of low redox potential; therefore, the increase in specific energy is not significant in most cases. For some organic electrodes, specific capacities in excess of 300 mAhg−1 can often be obtained; however, their redox potentials are mostly well below 3 V (vs. Li+/Li). Therefore, much work has been done to identify organic electrodes with high redox potentials. For example, a few p-type organic materials exhibit high redox potentials (vs. Li+/Li) exceeding 3.5 V, as shown by the triangular data points in Fig. 4a. In addition, the n-type material was chemically modified to increase the redox potential to a value comparable to that of transition metal-based materials. For organics, the redox potential of (2,5-dilithioxy)lithium terephthalate can be increased by as much as 800 by including bystander ions with higher ionic potentials than lithium, such as Ca2+, Ba2+ and Mg2+ mV.
Electrode-level specific energy evaluation of organic redox active materials
Since the discovery of organic redox-active materials with enhanced energy density, their use in practical battery systems has been daunting. When it comes to practical energy density, the weight and volume of redox-active components must also be considered. These ingredients, such as conductive agents and binders, are required in electrode fabrication to ensure mechanical stability and electrical conductivity. Due to the insulating properties of organic materials, organic electrodes generally require higher contents of conductive agents and binders. According to literature surveys, most organic electrodes reported so far contain 30–70 wt% conductive agent to compensate for the low electronic conductivity, so that organic electrodes can reach specific energies comparable to transition metal-based electrodes. This is in stark contrast to commercial electrodes, where these additives make up less than 5 wt% of the total electrode. This problem has become an obstacle for organic electrodes. Although many previous studies have evaluated the specific capacity, redox potential, or specific energy of organic electrodes, the critical assessment of electrode-level specific energy covering all electrode components has been rarely performed. Therefore, the reported specific energies of redox-active organic materials at the electrode and material levels were compared, highlighting the general deviation between the two (Fig. 4b). The X-axis is the specific energy calculated from the weight of redox-active materials suggested in the literature, and the Y-axis is the specific energy calculated from the weight of the entire electrode, including the redox-active material, conductive agent, and binder. The dotted line in Fig. 4b represents the point at which the material-level specific energy coincides with the electrode-level specific energy. Analysis shows that conventional transition metal-based electrodes deviate only slightly from this line. In contrast, most organic electrodes exhibit specific energies much lower than expected from the material level. Notably, organic 1 appears to retain a high specific energy at the electrode level. However, this specific energy degrades rapidly within a few cycles, implying that the content of redox-active components (conducting agents or binders) is too low for these components to perform their intended roles effectively.
Strategies to improve the specific energy of redox-active organic materials at the electrode level
Exploiting the potential high specific energy of organic materials at the electrode level is a necessary step for their practical application. As shown in Fig. 4c–e, certain strategies can close the gap and achieve high electrode-level specific energy. For example, the formation of charge-transfer complexes (Fig. 4c), when in a specific intermolecular arrangement that favors electron conduction, can reduce the amount of conductive agent required in the electrode due to the increased electronic conductivity of the complex. The charge-transfer complexes consisting of organics 3 and 10 (3-10 in Fig. 4b) or organics 3 and 11 (3-11 in Fig. 4b) exhibited significantly enhanced electronic conductivity over that of individual organics 3, 10, or 11. Inherent conductivity. Therefore, electrodes including one of these composites can be fabricated with more than 90 wt% active material, increasing the electrode-level specific energy. This type of approach, if developed further, would allow for high electronic conductivity while preserving the intrinsic redox activity of the two precursor organic materials. Likewise, linking a redox-active organic material (organic 13) into a covalent organic framework provides high electrical conductivity through π-π interactions of the dimly packed structure of the framework. Therefore, even with a small amount of conductive agent (less than 20 wt%), a high specific energy can be achieved at the electrode level, retaining almost the same specific energy as that at the material level.
High electrical order specific energies can also be achieved using multifunctional redox-active conducting agents with high electronic conductivity (Fig. 4d). For example, titanium disulfide (TiS2) not only exhibits a high electronic conductivity of 74.4 Scm−1, but can also contribute to the specific capacity through reversible intercalation. When the redox-active conductive agent in the electrode fabricated from organic 13 was partially replaced by TiS2, the resulting hybrid electrode could achieve a specific energy of 286 Wh per kg electrode, exceeding that of electrodes made with only conventional conductive agents (167 Wh per kg electrode) ).
Considering that conventional electrode fabrication processes have been optimized for commercial electrode materials (Fig. 4e), exploiting the unique properties of organic materials to design new electrode configurations may be another strategy. The unique properties of organic materials may offer unexplored benefits for electrode fabrication. For example, some molecules, such as organic 14, exhibit strong π-π interactions with graphitic carbon due to their polycyclic aromatic hydrocarbon-containing molecular structure. Molecular interactions with carbon-based conductive agents lead to well-connected electrode structures even with small amounts of carbon additives. In addition, the CNT additives further facilitated the electrical connection between organic particles in the electrodes because of their higher aspect ratios than the spherical carbon additives. Through a combination of the above methods, an electrode with 90 wt% active material was fabricated with microporous redox-active organic polymer 15 and single-walled CNTs, providing an enhanced electrode-level specific energy. Typically, a thick electrode is fabricated using traditional slurry casting methods, which can lead to cracking and delamination due to the mechanical instability of the electrode. However, the interaction between carbon additives (such as carbon nanotubes) and microporous polymers resulted in well-connected and highly loaded electrodes (Fig. 4e). Further efforts should optimize the fabrication process of organic electrodes so that the specific energy at the electrode level is consistent with that at the material level (shown by the dashed line in Fig. 4b).
Comparative analysis of specific power
Batteries require high power capability in order to generate a large amount of energy in a short period of time and to make the charging process faster. Although the most intuitive way to achieve high power is to employ electrode materials with fast ion and electron transfer capabilities, this remains challenging for most organic electrodes due to their generally low electron conductivity. In Fig. 5a, representative high-power organic electrodes are selected and their normalized rate capabilities compared to conventional inorganic electrodes are plotted.
Analysis shows that radical polymer electrodes, such as electrodes with (2,2,6,6-tetramethylpiperidin-1-yl)oxygen units (Organic 35) exhibit competitive rate capabilities up to near 20C, which is comparable to transition metal-based materials. Furthermore, as shown by the circular data points in Fig. 5a, the organic 16-based radical polymer electrode provided 46% of the theoretical capacity in less than 1 minute at charge and discharge (100C), demonstrating its excellent kinetic capability This power capability can be attributed to the high electronic conductivity of radical polymers, which arises from an electron hopping mechanism through electron self-exchange.
Strategies to improve the rate capability of redox-active organic materials
To improve the performance, a charge-transfer complex composed of two redox-active organic materials 3 and 25 (3–25 in Fig. 5a) was produced and exhibited remarkable capacity utilization at 965 mAg−1, equivalent to that in 76% retention of the capacity provided at 48 mAg−1. This performance stems from the enhanced electronic conductivity of the charge-transfer complex, which, as discussed previously, facilitates the transport of charges in the rearranged molecular structure. Compared with the pristine constituent materials (3 and 25), the electronic conductivity of the 3-25 charge-transfer complex was increased by more than 10,000 times.
Sulfur-substituted dicarboxylate compounds, such as organic 5, can also deliver a respectable specific power of 909 watts per kilogram of material and a capacity of 176 mAhg−1 at 4.2C. Although this performance is not significant, it represents a substantial improvement over the 225 watts per kilogram of material of the original dicarboxylate engine. It is speculated that replacing oxygen with sulfur in the diformate-based pattern can increase the electronic conductivity to about 10 Scm, because sulfur’s higher electronic polarizability increases the power capability by a factor of nearly 10.
Although most redox-active organic materials exhibit lower electronic conductivity (less than 10 Scm−10) relative to transition metal-based materials (about 10 Scm−1), structural or chemical modifications can lead to higher power capabilities. Figure 5a demonstrates the rate capability of common redox-active organic materials whose specific powers have been enhanced by various engineering approaches. Morphological control of organic particles has been shown to be effective. For example, modifying the shape of the organic matter 32 to a sheet shape instead of a spherical shape, or reducing the particle size of the organic matter 36 to a nanometer scale will increase the contact area with the conductive agent. This enhanced contact area enables efficient electron absorption from the conducting agent and reduced ion diffusion paths, resulting in a successful increase in rate capability, as shown by the triangular data points in Fig. 5a. Furthermore, hybrid structures employing advanced carbon materials can achieve high power by exploiting the ultra-high electrical conductivity (above 104 Scm−1) of graphene or CNTs. Electron transport into and out of redox-active organic materials can be tailored through pathways connected by interactions between π systems in organic frameworks and carbon materials, leading to increased rate capability. Using this strategy, various organic electrodes, including those with organics 17, 28, 32, 35, and 37, can successfully deliver appreciable specific energies that are comparable to inorganic electrodes even at discharge rates of 10C or higher. Compare.
Electrode-level specific power evaluation of redox-active organic materials
Using the method outlined in Figure 5a, the power capability is greatly improved; however, organic electrodes still require a large amount of conductive agent, resulting in lower specific power and energy at the electrode stage. Figure 5b demonstrates the rate capability of the aforementioned redox-active organic materials based on electrode-scale capacity. Even though aggressive polymers exhibit power capabilities that compete with inorganic materials (Fig. 5a), the actual rate capability is far from the delivery at the material level considering the weight of the entire electrode. Fabricating electrodes with large amounts of advanced carbon materials may not be the best strategy for high-performance organic rechargeable batteries because of the consequent reduction in volumetric and gravimetric energy and power density. The field of organic batteries is still waiting for a solution to achieve high power density without sacrificing energy density, possibly by combining strategies at the material and electrode levels.
Comparative analysis of cycle life
The cycle life of organic rechargeable batteries is mainly constrained by common factors, such as electrolyte and electrode aging and degradation due to repeated electrochemical reactions, which are also responsible for the cycle stability of conventional Li-ion batteries. However, several properties of organic materials are particularly critical to the cycling stability of organic rechargeable batteries, such as the structural integrity of organic crystals. Unlike transition metal-based electrode materials, organic crystals are held together by weak intermolecular van der Waals forces. Therefore, redox-active organic materials tend to dissolve easily in liquid electrolytes and can fall off the electrodes by the dissolution of solvent molecules in the electrolyte. The continuous loss of redox-active organic materials from the electrodes leads to a gradual decrease in capacity upon cycling, which is a key hurdle limiting its practical application. Furthermore, the organic crystal structure can easily become disordered and degraded with repeated insertion and extraction of guest ions, leading to undesirable changes in electrochemical activity.
Strategies to increase the cycle life of redox-active organic materials
Many strategies have addressed these issues and improved loop stability. In Fig. 5c, we compare and summarize the cycling performance of redox-active organic electrodes obtained by various strategies, namely the reported number of cycles and capacity retention. One of the most intuitive examples includes dimer/trimer and/or polymer-based organic species (represented by pentagonal data points), such as organic 31 and organic polymer 16, which exhibited Up to 93% cycle stability35. When individual redox patterns are anchored to each other by covalent bonds, the enlarged size of the molecules hinders the dissolution process, reducing their tendency to be dissolved. In another example, anthraquinones with high solubility, which have poor cycling performance in their molecular forms, were tailored into polymeric forms (organic 38). Further addition of advanced carbon materials to the electrodes brought about 57% capacity retention after 9000 cycles (organic 15). Although polymerization improves the cycle life of organic electrodes, there is also a clear trade-off; polymerization results in poor rate capability, which stems from the low electronic conductivity of common polymers and reduced capacity due to the addition of redox-active frameworks. To address these tradeoffs, some new polymers are worth noting. For example, a ladder-type polymer (organic 22), synthesized in a configuration that minimizes redox active components, achieved high capacity and long cycle life. A donor-acceptor polymer (Organic 39) directly links the electron-donor polymer backbone with electron-acceptor groups while achieving high cycling stability and rate capability. The use of advanced carbon materials is another approach to ensure cycling stability, as they stably immobilize redox-active organic materials through π-π interactions, preventing dissolution. These methods are effective for redox active compounds including organics 3-10, 14, 15, 17, 28, 35 and 37. The dissolution problem of organic electrodes can also be solved by designing electrolyte properties. Since free solvents in electrolytes can trigger the dissolution and dissociation of organic compounds, attempts have been made to reduce or eliminate free solvents in electrolytes. One way to reduce the effect of free solvents is to use high concentrations of electrolytes. Because the number of free solvent molecules is greatly reduced with the change of the dissolved structure in the high-concentration electrolyte, the dissolution of organic electrodes is subsequently hindered. Small-scale oxygen-reduction-active organic materials, such as organics 13, 20, and 40, are able to maintain stable cycling performance without any molecular engineering due to the suppressed dissolution in high-concentration electrolytes, Figure 5c. The use of solvent-free electrolytes, such as ionic liquids, is also a promising route to inhibit dissolution. For example, electrodes made of organic 18 (Calix[6]quinone) can exhibit 69% capacity retention after 1000 cycles with the ionic liquid N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)amide. This performance represents an important improvement over the conventional electrolyte system (ethylene carbonate/dimethyl carbonate with 1M lithium hexafluorophosphate), which can only provide 53.7% of the initial capacity after 100 cycles. Likewise, polymer or plastic crystalline-type electrolytes can support the cycling stability of redox-active compounds, such as organics 18, 19, 37, and 41. Low molecular mobility in these systems can hinder the free movement of molecules, inhibiting undesired dissolution. Furthermore, the introduction of inorganic solid electrolytes, a new electrolyte type investigated for Li-ion battery research, is an effective countermeasure to exclude the dissolution of redox-active organic materials. Although the latter method is still in the early stages of research and has only been used for organics 30, 42 and 43, its clear advantages, especially the prevention of the dissolution of organic materials, may open up new opportunities for organic electrode materials.
The capacity drop can be attributed not only to the dissolution of the organic electrode itself, but also to side reactions caused by the crossover of dissolved species in electrochemical cells, leading to passivation or shuttle effect of the counter electrode, where the dissolved species are between the anode and cathode. Move, repeat oxidation and reduction. The transport of dissolved redox-active organics to the counter electrode can be suppressed by rational membrane engineering.
A simple approach is to coat the commonly used polypropylene or polyethylene separator with an additional layer, such as graphene or polyimide, to modulate transport through the separator, since the additional layer allows small lithium ions and electrolyte penetration, but Usually inhibits the penetration of organic matter. The organic electrodes from organics 3, 13, 26, and 44 can achieve enhanced cycling stability by employing coated separators compared to bare separators under the same electrode and electrolyte conditions. By using the Nafion-coated separator, the electrode with organic 44 was able to retain 76% of the initial capacity after 400 cycles, while using the bare polypropylene separator only retained 64% of the capacity after 50 cycles. A novel fully selective membrane is also proposed, with new membrane engineering allowing precise control of pore size to selectively block redox-active organic molecules while transporting ions in the electrolyte. In this regard, taking advantage of the nanopores of metal-organic framework (MOF) materials, a monolithic gel-type membrane with fully selected properties was synthesized. Pores in MOF materials can be tuned to the desired size and distribution, allowing custom-designed membrane separators for target organic molecules. By employing a MOF-gel separator based on zinc and 2-methylimidazole, a model organic compound, the quinone derivative organic 45, exhibits remarkable cycling stability after 2000 cycles at a current density of 269 mAg−1, The retention rate is about 83%.
Alternative energy storage platforms
The suitability of redox-active organic materials is often investigated in the configuration of lithium-ion batteries. However, despite considerable efforts, the application of redox-active organic materials still has some unresolved issues, such as the inevitable solubility in electrolytes and low power density, which can only be improved by greatly sacrificing energy density. Efforts to produce battery platforms other than lithium-ion batteries (so-called post-lithium-ion batteries) have opened up new opportunities for redox-active organic materials. Due to their diverse physical and chemical structures, these organic materials are easier to apply to these new platforms than conventional transition metal-based electrode materials.
Earth-abundant metal-ion organic rechargeable batteries
Concerns about the availability of lithium feedstocks have prompted the exploration of battery chemistries based on earth-abundant metal ions, such as sodium, potassium, magnesium, calcium, and aluminum. Batteries based on sodium ions and potassium ions are the most widely studied, as the operating mechanism of monovalent ion systems is expected to be similar to that of lithium ion batteries. Furthermore, they share several important electrode material groups, such as AxMO2 (A=Li, K or Na; M=transition metal), which allow not only the reversible intercalation of lithium ions but also sodium and potassium ions. However, using larger ions such as Na+ (1.02 Å) and K+ (1.38 Å) than Li+ (0.76 Å) tends to involve a greater alternation of crystal structures, which requires larger volume changes in the electrodes and intercalation hosts faster degradation. Unlike the rigid structure of traditional inorganic intercalation hosts, redox-active organic materials provide a more flexible framework. Since organic crystals are usually bound by weak van der Waals forces, compared with rigid inorganic compounds, a space large enough to accommodate various guest ions can be provided, making redox-active organic materials less susceptible to charge-carrying ions size effect. This feature enables the stable insertion of larger guest ions such as Na+ and K+ into various redox-active organic compounds, as shown in Figure 6a. For example, organic 13 can reversibly store Na+ and K+ ions, utilizing two carbonyl motifs in the structure. Li, Na, and K half-cells with organic 13 electrodes can deliver specific capacities of 127, 107, and 105 mAhg−1, respectively, indicating that the choice of metal has no significant effect on the specific capacity. In addition, considerable cycling and power performance can be achieved in Na and K batteries, which can provide about 94% and 87% of the initial capacity after 200 cycles and 1000 cycles, respectively, while Na battery at 895mAg−1, K battery at 895mAg−1 4,475mAg−1, 81mAhg−1, 70mAhg−1 can be reserved. These results are in stark contrast to typical inorganic hosts, whose intercalation abilities vary widely depending on the species of guest ions. Likewise, given its azo redox theme, organic 50 can store two equivalent ions in Li, Na, and K half-cells. All half-cells exhibited high capacity retention, suggesting that redox-active organic materials are less susceptible to the type of guest ions. Furthermore, organic 49 provides a high capacity of 232 mAhg−1 based on the full utilization of all four carbonyl groups and maintains 97% of the initial capacity after 10,000 cycles, which is considered to be an outstanding stability for Na-ion battery cathodes.
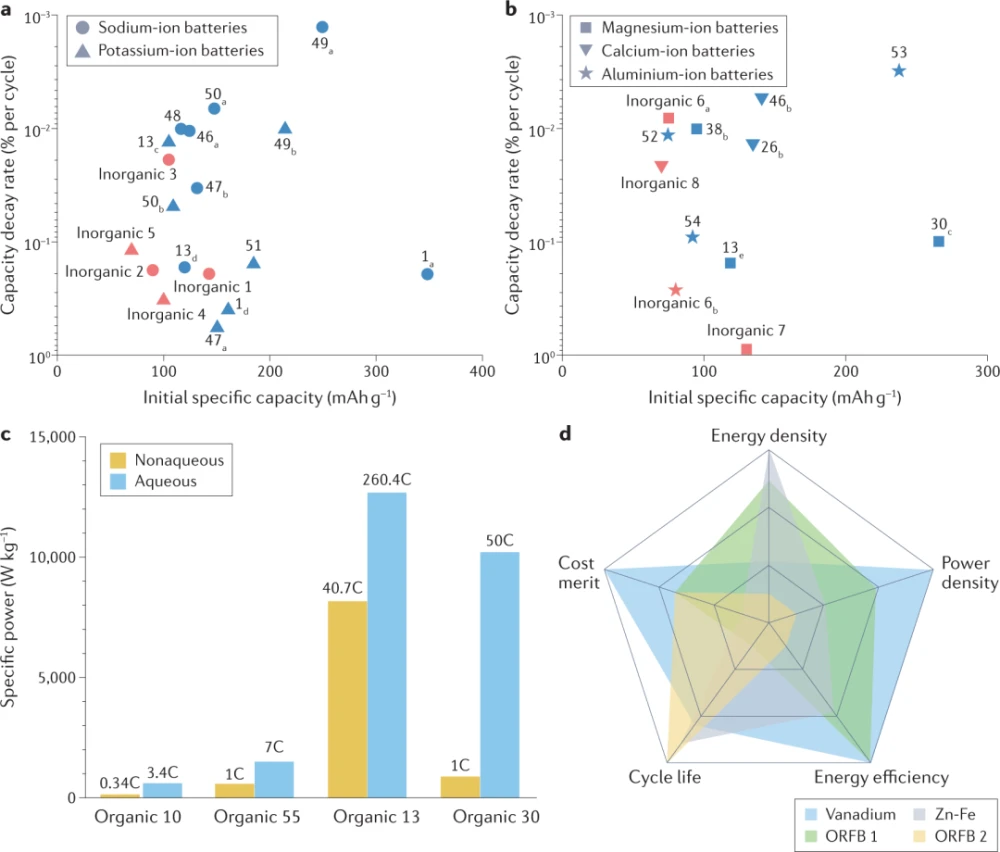
Figure 6: Potential for post-Li-ion battery configuration with redox-active organic materials.
Redox-active organic materials can also be successfully used in multivalent ion batteries. Despite the potential advantages of multivalent ion battery systems, the lack of suitable hosts for multivalent ions remains one of the key obstacles. One of the major problems is the severely sluggish diffusion kinetics typically observed in intercalated hosts of most multivalent ions. Therefore, only a few inorganic host materials have been reported so far, including Chevrel phases (Mo6S8, Mo6Se8) and vanadium oxides (V2O5, VO2) for magnesium or aluminum-ion batteries. Although their development is still in its infancy, several redox-active organic materials have been identified as promising cathode materials for multivalent ion batteries, as shown in Fig. 6b. Organic 30, containing four carbonyl redox groups, can deliver a remarkably high capacity of 266 mAhg−1, which is equivalent to inserting two Mg2+ ions in one magnesium half-cell. Furthermore, 82% of the initial capacity was successfully retained even after 200 cycles, which is almost comparable to the performance observed in lithium batteries. Multivalent aluminum complex ions can be reversibly stored in triangular macrocycle organics 52, which form a layered organic crystal structure. The intercalation and extraction of cationic aluminum complexes can achieve a reversible capacity of 75 mAhg–1 with a cycleability of up to 5,000 times. Synthesis of macrocycle organics 53 with a reduced ratio of benzene rings to carbon groups also resulted in high-capacity aluminum-ion battery cathodes. The divalent ion (AlCl2+) is easier to store than its competing monovalent ion (AlCl+), resulting in a higher specific capacity of 237mAhg−1. In this regard, true multivalent aluminum-ion batteries based on organic active materials can be realized, unlike previously reported systems that rely on monovalent aluminum complex ions for the main redox reaction.
Water-based organic rechargeable battery
The electrolytes currently used in lithium-ion batteries are flammable and toxic. Aqueous rechargeable batteries can circumvent these problems while offering much higher ionic conductivity (about 1 Scm−1) than typical organic-based electrolytes (10−3–10−2 Scm−1). Therefore, their use may help to compensate for the low kinetic properties of redox-active organic materials. In this regard, some attempts have been made to achieve high power capability of organic electrodes in aqueous battery systems, as shown in Figure 6c. For example, when organic 13 was used as an electrode in a sodium half-cell with ethylene carbonate/diethyl carbonate electrolyte, only 65% of the capacity was provided when the current rate was increased from 0.07C to 7C. In contrast, when a 2M zinc chloride (ZnCl2) aqueous electrolyte is employed in the zinc half-cell 209, the same electrode can maintain nearly 62.6% capacity when the current rate is increased from 1.6C to an extremely high value of 260.4C. The system has a specific power of 12.7 kilowatts per kilogram of material, which is even higher than conventional lithium-ion batteries (about 1 kilowatt per kilogram of material). In addition, the organic 30 with 2M zinc sulfate (ZnSO4) aqueous electrolyte exhibited a specific power of 10.2 kW per kg of material in a zinc half-cell at 50C, which far exceeds that of the same organic in 1M lithium hexafluorophosphate and ethylene carbonate/diethyl carbonate A specific power of 0.83 kilowatts per kilogram of material was obtained when the composition was utilized in a non-aqueous system.
Although aqueous electrolytes can provide extraordinary kinetic capabilities for redox-active organic electrodes, their introduction leads to a narrow electrochemical window (theoretically 1.23 V for water splitting). The narrow electrochemical window not only results in a limited available energy density, but also leads to various side effects such as gas swelling during repeated cycling. Employing high-concentration electrolytes, so-called water-in-salt electrolytes, is one of the strategies that might overcome these drawbacks. In the water-in-salt system, the electrochemical window is enlarged due to its unique dissolution structure, which leads to a decrease in the electrochemical activity of water. The concentrated brine electrolyte containing 30 M potassium bis(fluorosulfonyl)imide effectively suppressed the evolution of hydrogen, extending the electrochemical window to nearly 4 V. Therefore, organic 13 can serve as a stable anode for aqueous potassium batteries with better cycling stability. Furthermore, the dissolution of redox-active organic materials can be greatly suppressed in brine electrolytes.
With the continuous development of the water-based organic rechargeable battery field, effective methods in the application of water-based inorganic batteries should be explored. For example, confinement of the water solvent within the polymer network reduces water activity, while spatially separated acid-base bielectrolytes amplify the electrochemistry of full cells by reducing the hydrogen evolution reaction potential and increasing the pH-based oxygen evolution reaction potential window. Applying this approach to aqueous organic batteries should help overcome concerns about low energy density.
Organic Redox Flow Batteries
Redox flow batteries, which rely on solution-based redox chemistry of active materials, often employ soluble inorganic redox couples such as V/V, Zn/Fe, or Zn/Br. Given concerns about their highly unstable price and toxicity, there is growing interest in replacing these redox pairs with redox-active organic materials. Redox-active organic materials are advantageous over their inorganic counterparts not only because of their potential cost-effectiveness, but also because of their chemical tunability, which enables targeted electrochemical performance by tuning solubility or molecular size. In Fig. 6d, we compare performance and cost metrics such as energy density, power density, energy efficiency, cycle life, and cost of organic redox flow batteries with reference to representative inorganic redox flow batteries. In the figure, the scores for each evaluation item are normalized according to the maximum and minimum values of each item, and these scores are obtained from previous studies on inorganic vanadium-based and zinc/iron-based redox batteries and organic 1, 8-Bis(2-(2-(2-hydroxyethoxy)ethoxy)-ethoxy)anthracene-9,10-dione/ferricyanide (ORFB 1)-based and ((9,10 -Dioxy-9,10-dihydro-2,6-diyl)bis(oxy))bis(propane-3,1-diyl))bis(phosphonic acid)/ferrocyanide(ORFB 2)-based redox-flow batteries .
As can be seen in Figure 6d, the performance of organic redox flow batteries can complement some aspects of representative inorganic-based redox flow batteries.
In terms of energy density, designing solubilizing groups is an effective strategy to improve the solubility of active materials, thereby increasing the volumetric specific capacity of organic redox flow batteries. Introduction of non-ionic polyethylene glycol-based functional groups into anthraquinone yields water-soluble 1,8-bis(2-(2-(2-hydroxyethoxy)-ethoxy)anthracene-9,10-di ketone, providing ORFB1 with a volumetric capacity of 80.4Ah per liter and an energy density of 25.2Wh per liter. The molecular structure of a representative ferricyanide anion can be redesigned by breaking the symmetry of its ligand, where hydrophilic Replacing the cyanide ligands with the bipyridine ligands can make it soluble in water up to 1.09M, while the solubility of sodium ferricyanide is 0.62M. In addition, the redox potential of sodium ferricyanide can be changed from 0.26V (vs. Ag /AgCl) to 0.65 V (vs. Ag/AgCl) because the interaction of bispyridine ligands with iron centers is weaker than that of cyanide ligands with iron centers.
The low cycle life, mainly due to the instability of redox-active organic materials in the charged state, is the main bottleneck hindering the practical use of organic redox flow batteries. Since molecules in solution can interact with other elements relatively freely, various side reactions, such as dimerization or nucleophilic attack from solvent molecules, may occur more easily than in solid-state materials. These adverse reactions reduce the amount of active material and subsequently shorten the lifetime of redox flow batteries. However, it is possible that these limitations can be overcome. For example, more positively charged functional groups can inhibit the radical dimerization of ferrocene derivatives and organic 56 in the charged state through electrostatic repulsion. Redesigning the redox-active organic material also mitigated the crossover phenomenon known to greatly contribute to cycle degradation, resulting in a capacity retention rate of 99.9943% per cycle and 99.967% per day. Furthermore, the study of the degradation mechanism of carboxyanthraquinone has inspired molecular redesign of carboxyanthraquinone to phosphonate anthraquinone, limiting carboxylate-mediated intramolecular nucleophilic substitution. This suggests that redox-active organic materials are rapidly approaching the level required for practical application of redox flow battery systems.
Outlook
Growing concerns about global environmental pollution have sparked the development of sustainable and eco-friendly battery chemistries. In this regard, organic rechargeable batteries are considered as promising next-generation systems that can meet the needs of this era.
For the practical realization of organic rechargeable battery systems, the performance indicators at the electrode level should be evaluated more carefully and eventually extended to the evaluation of performance indicators at the battery level. That said, the type or amount of electrolyte should also be considered in order to achieve high gravimetric or volumetric energy densities, although such performance metrics have not yet been established. In addition, the fundamental understanding of the compatibility of organic electrodes with electrolyte systems should be explored when performing optimization procedures. The type of energy storage platform that most effectively exploits the benefits of redox-active organic materials should also be considered. For example, organic rechargeable batteries are often disadvantageous in terms of volumetric energy or power density because of their inherently low density. To build practical organic batteries, innovative electrode engineering and system design are necessary. Another strategy is to build targeted applications where environmental friendliness and cost-effectiveness are critical, such as stationary energy storage systems, which typically store large quantities of intermittent renewable energy, rather than applied to mobile devices.
Redox-active organic materials are considered eco-friendly because they are usually produced under mild synthetic conditions, have a low environmental impact, and can even be obtained from natural products. Nonetheless, since the production and recycling process of redox-active organic materials has not been thoroughly studied, it can be said that the sustainability of redox-active organic materials is only a conjecture. However, preliminary steps to recover redox-active organic materials have been taken to facilitate the recyclability of decomposition products. While this strategy must be further developed to fully recycle such batteries, the possibility of recycling redox-active organic materials improves the prospects for organic rechargeable batteries for next-generation energy storage. Future efforts should also investigate how to produce forward-looking organic electrode materials with a minimal environmental footprint. Furthermore, given that traditionally used battery components, such as binders and electrolytes, are often either environmentally friendly or not based on sustainable chemistry, similar attention should be paid to other components that make up organic rechargeable batteries. With proper selection or adjustment of redox active organic materials, these components can be replaced with more environmentally and economically viable options. With continued and consistent efforts to improve the performance and sustainability of organic batteries, a greener, rechargeable world may not be far off.

